Indications and Usage
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Important Safety Information
Altreno is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Skin Irritation: Patients using Altreno may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of Altreno, or discontinue use. Avoid application of Altreno to eczematous or sunburned skin.
Ultraviolet Light and Environmental Exposure: Minimize unprotected exposure to ultraviolet light, including sunlight and sunlamps. Warn patients with frequent sun exposure and those with inherent sensitivity to sunlight to exercise caution. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided.
Fish Allergies: Altreno contains soluble fish proteins. Use with caution in patients with known sensitivity or allergy to fish. Advise patients to contact their healthcare provider if they develop pruritus or urticaria.
Adverse Reactions: The most common adverse reactions in clinical trials were application site dryness (4%), pain (3%), erythema (2%), irritation (1%) and exfoliation (1%).
Nursing Women: It is not known whether topical administration of tretinoin could result in sufficient systemic absorption to produce detectable concentrations in human milk. The developmental health benefits of breastfeeding should be considered along with the mother’s clinical need for Altreno and any potential adverse effects on the breastfed child from Altreno.
To report suspected adverse reactions, contact Customer Service at 1-800-321-4576 or the FDA at 1-800-FDA-1088 or fda.gov/medwatch.
Indications and Usage
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Indications and Usage
Important Safety Information
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Important Safety Information
Altreno is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Skin Irritation: Patients using Altreno may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of Altreno, or discontinue use. Avoid application of Altreno to eczematous or sunburned skin.
Ultraviolet Light and Environmental Exposure: Minimize unprotected exposure to ultraviolet light, including sunlight and sunlamps. Warn patients with frequent sun exposure and those with inherent sensitivity to sunlight to exercise caution. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided.
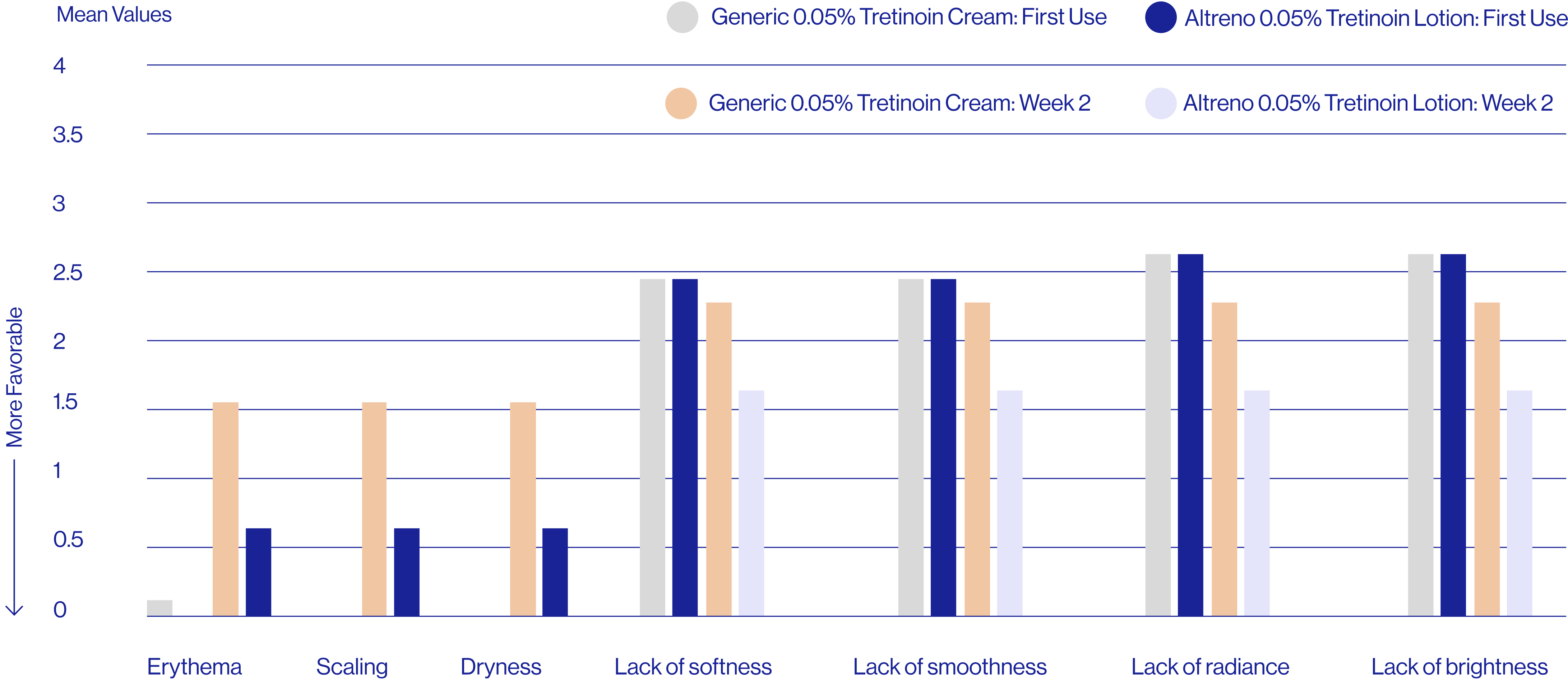
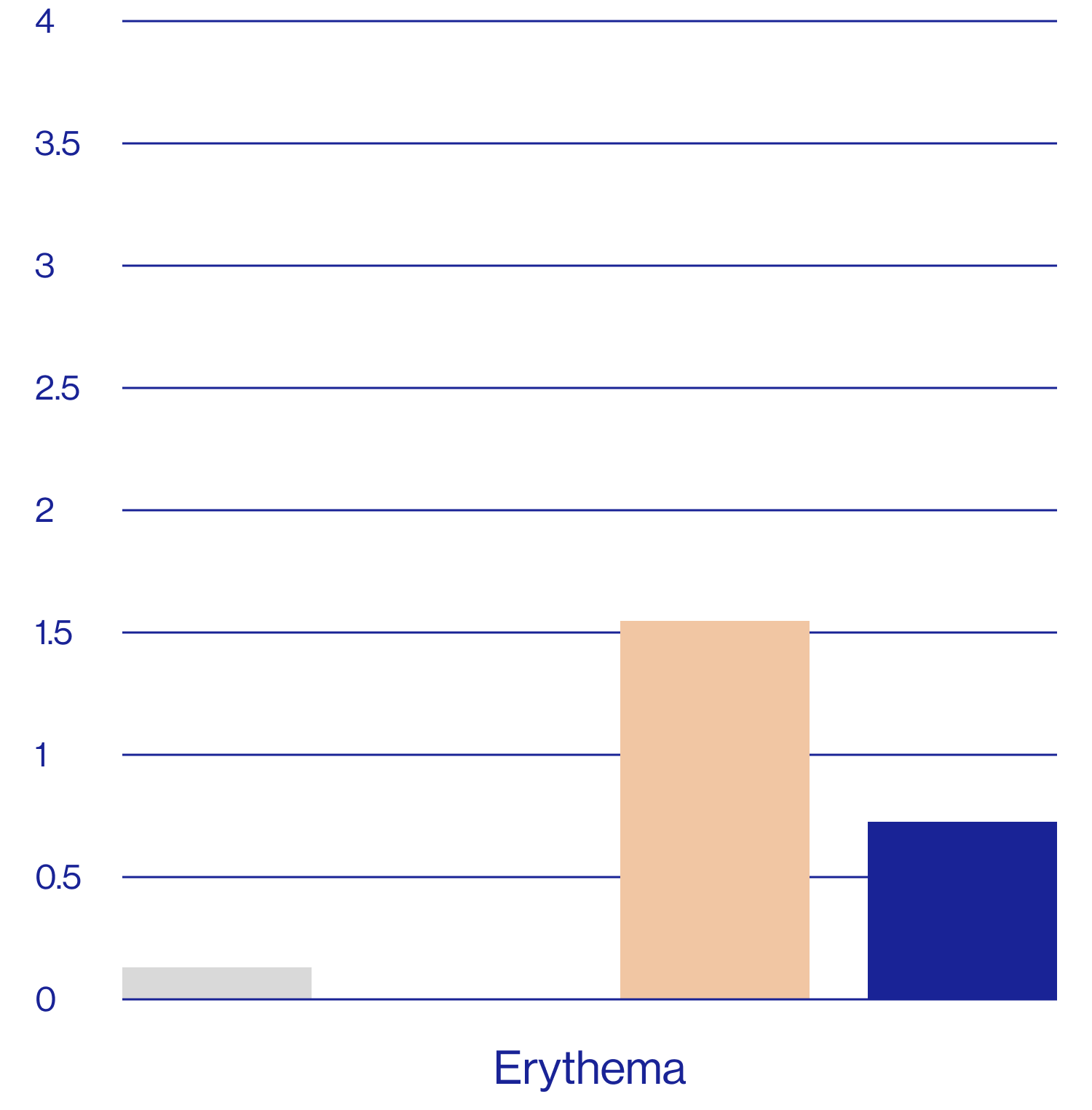
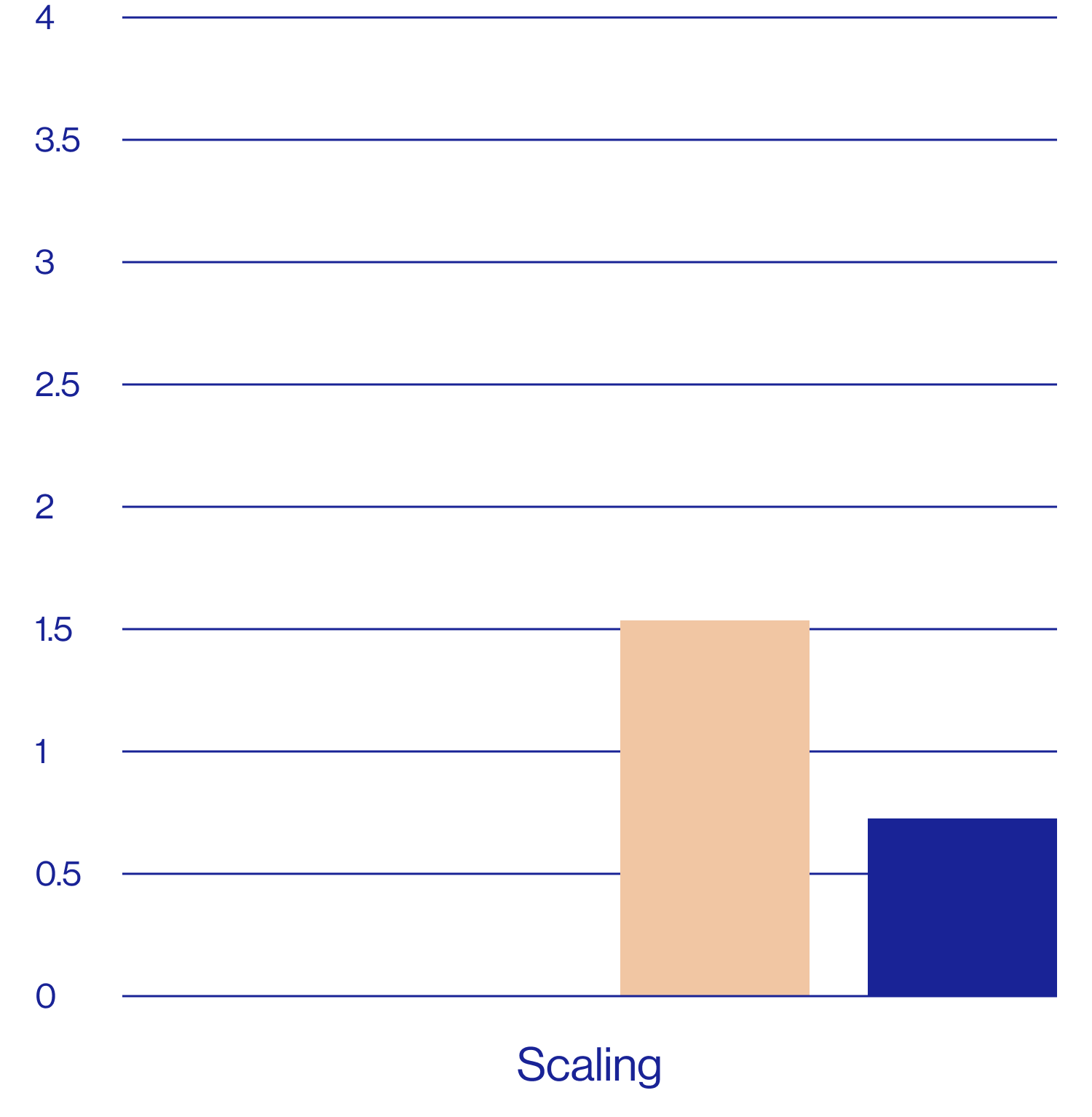
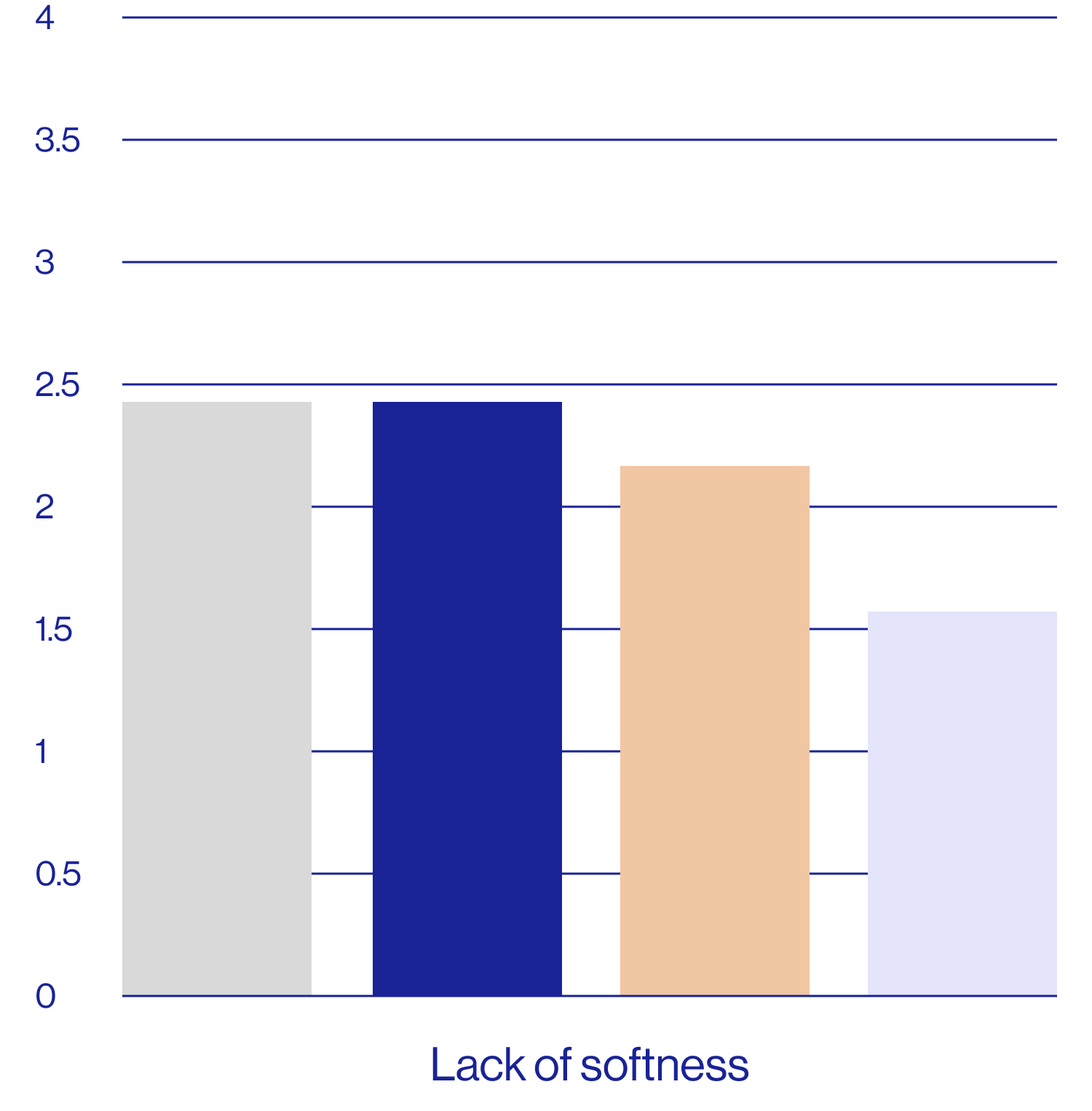
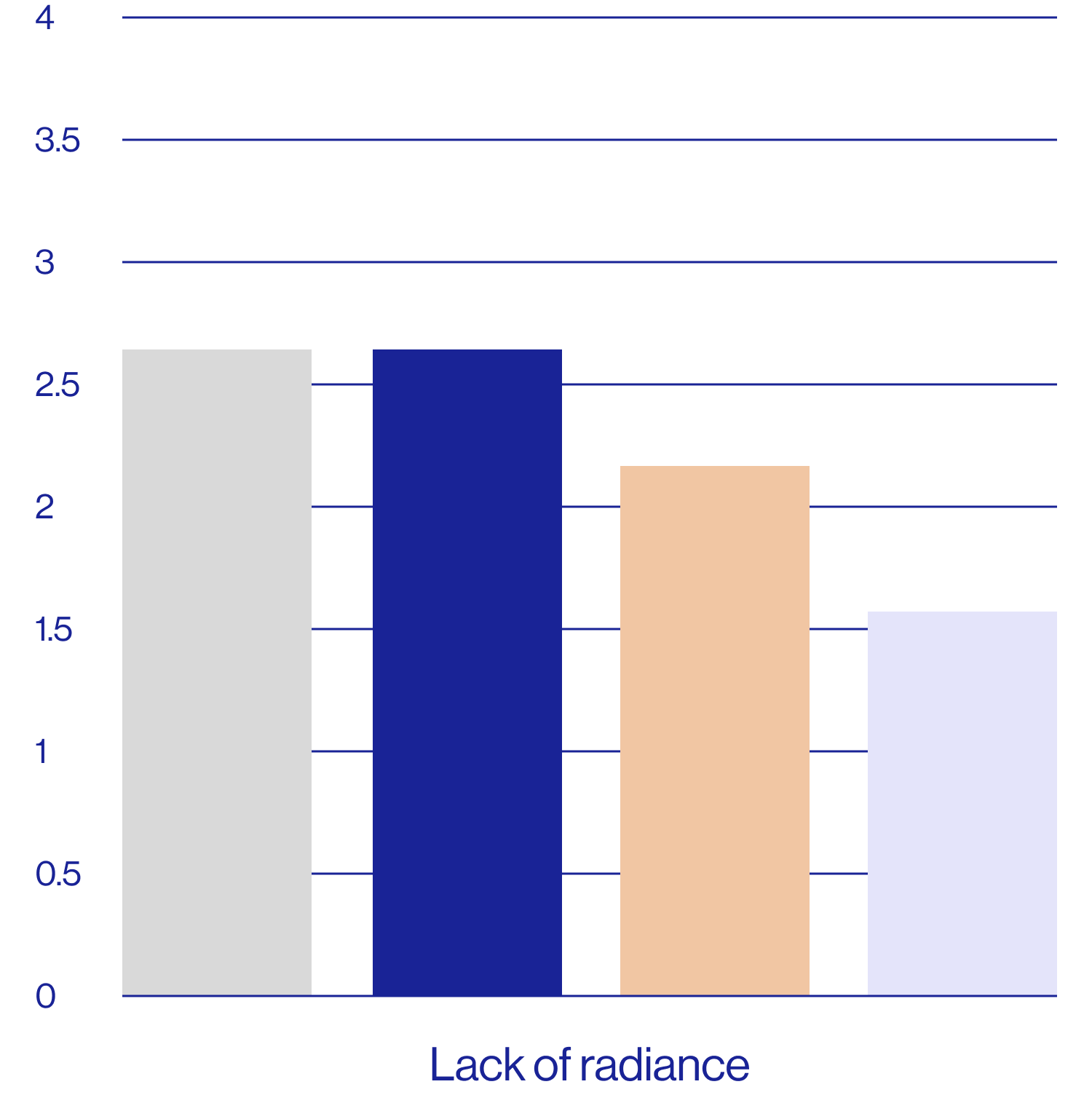
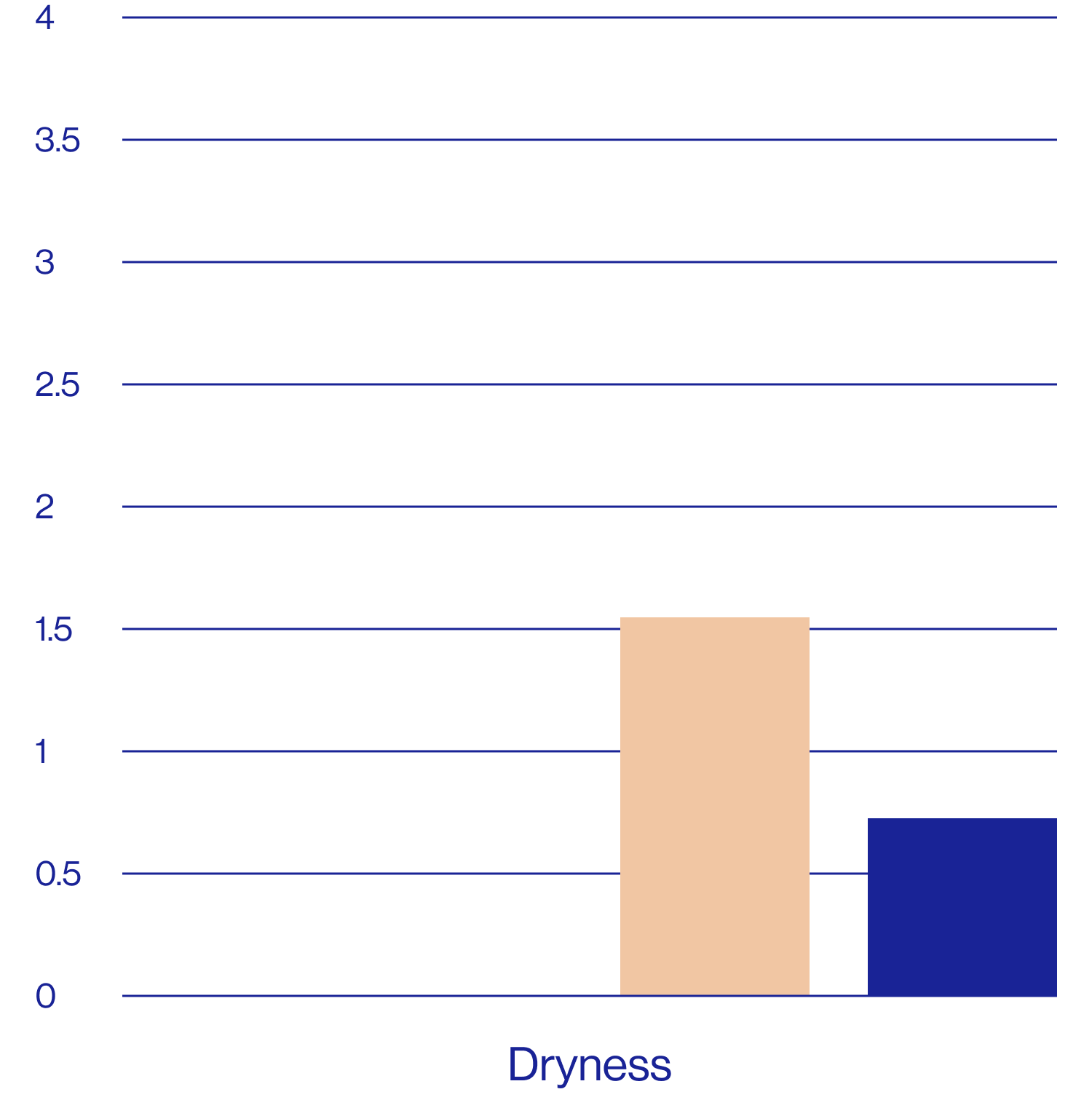
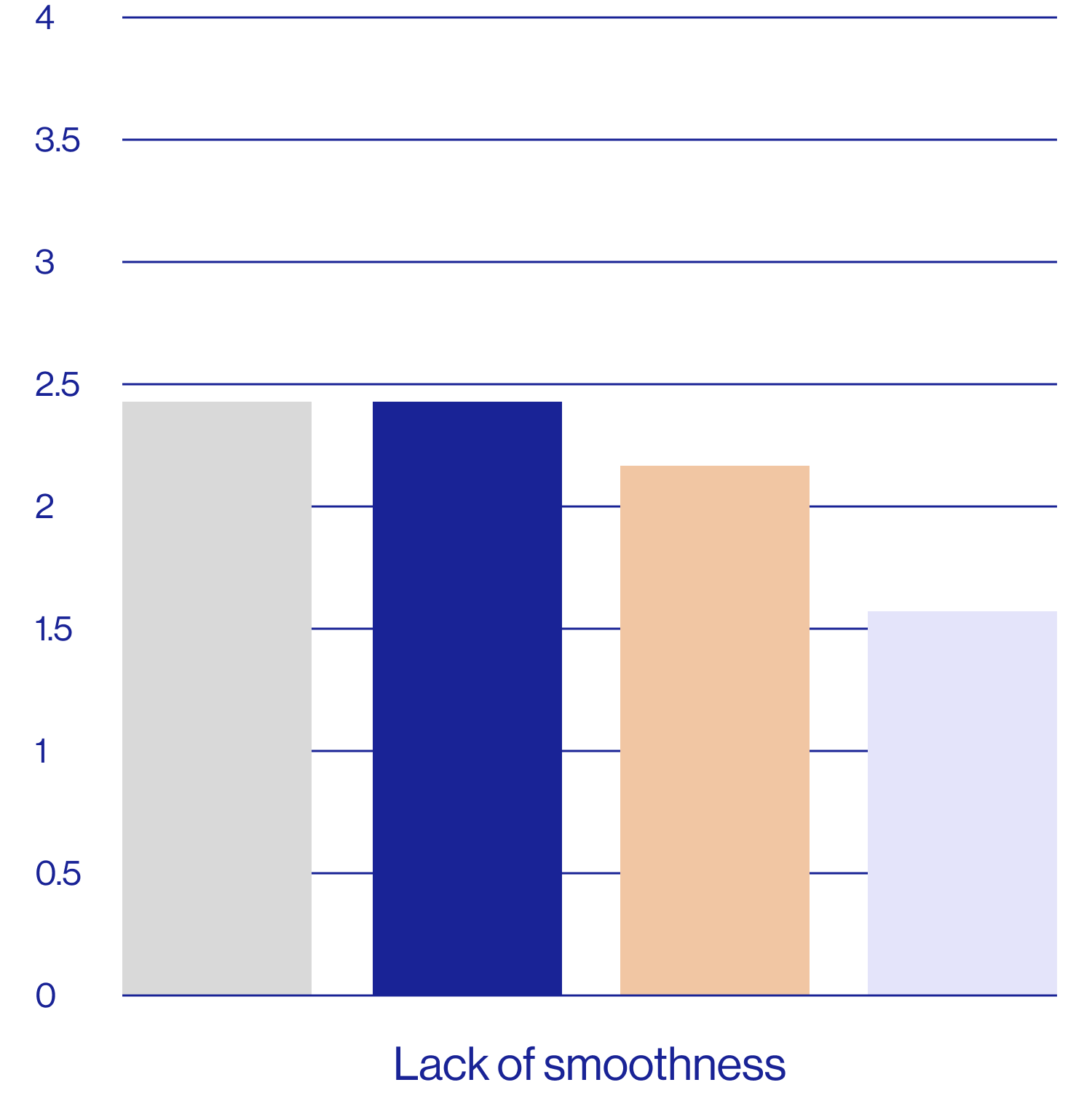
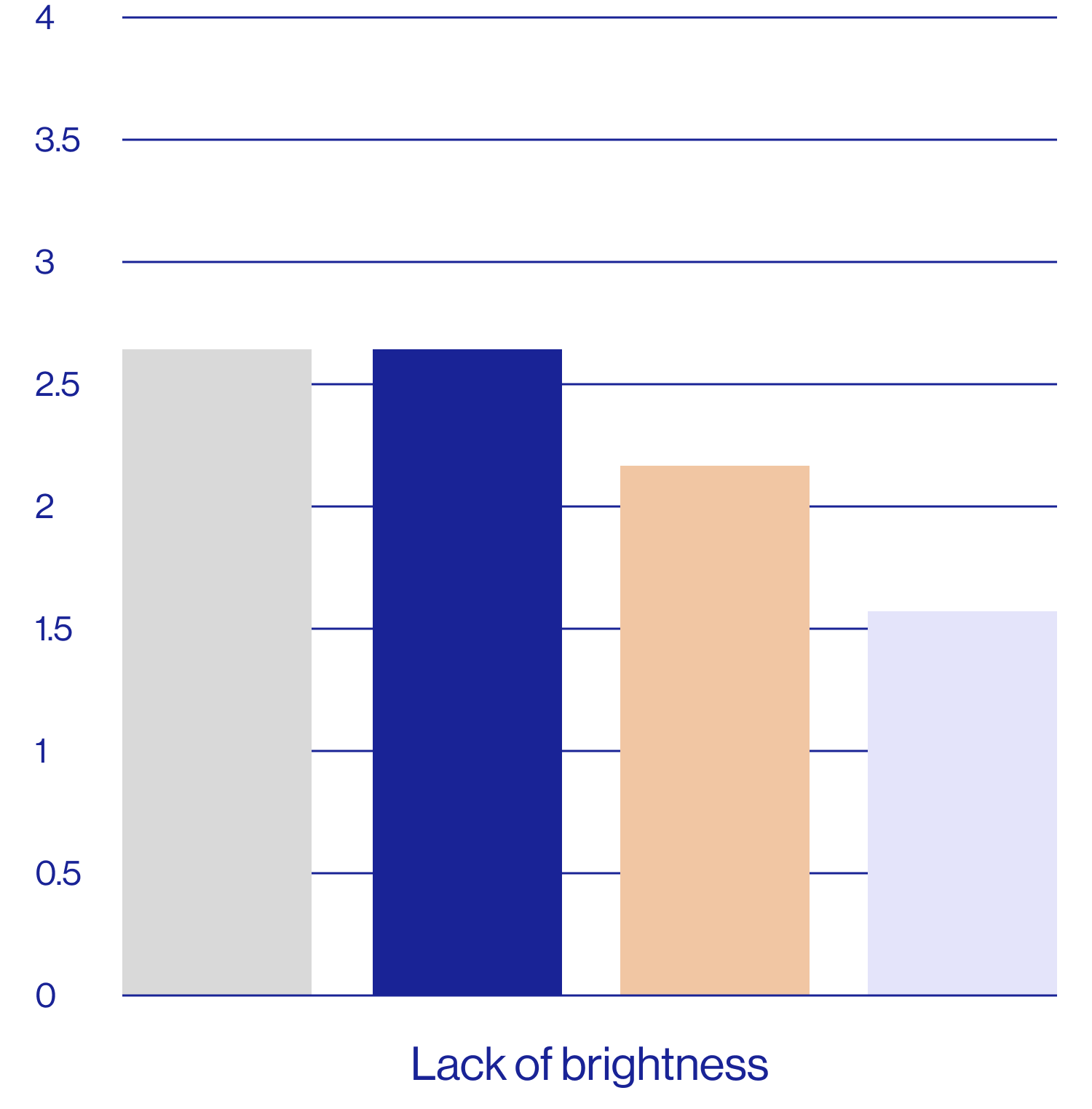
Patient self-assessments of skin properties at week 2 (N=25)
|
Altreno Lotion Generic Cream
|
More Agreement More Disagreement More More |
% Agreea Altreno |
% Agreea Generic |
|---|---|---|---|
| Feels soft | 2.64 5.04 | 80% | 32% |
| Feels smooth | 2.60 4.84 | 80% | 40% |
| Feels comforted/ soothed/calmed |
3.28 5.52 | 72% | 24% |
| Does not feel dry | 3.40 5.80 | 68% | 28% |
| Looks smoother | 3.20 5.60 | 76% | 28% |
| Looks less dull | 3.16 5.52 | 72% | 28% |
| Looks less flaky | 3.60 6.48 | 64% | 20% |
| QUESTIONNAIRE SCALE Questionnaire scale |
1 2 3 4 5 6 7 8 9
|
||
Questions rated on a scale of 1=agree completely to 9=disagree completely.
aDefined as a rating of 1, 2, or 3 on the questionnaire scale.
The patient self assessment questionnaire is not a validated tool and therefore results are exploratory and should be interpreted with caution.
Patient self-assessments of product properties at week 2 (N=25)
|
Altreno Lotion Generic Cream
|
More Agreement More Disagreement More More |
% Agreea Altreno |
% Agreea Generic |
|---|---|---|---|
| Feels gentle | 2.52 5.12 | 84% | 32% |
| Feels comfortable/ soothing |
2.64 4.72 | 80% | 36% |
| Relieves tightening sensation | 3.16 5.60 | 64% | 24% |
| Spreads well | 1.64 7.32 | 92% | 8% |
| Absorbs well | 2.12 6.40 | 88% | 20% |
| Is not sticky | 3.52 6.08 | 72% | 28% |
| Leaves minimal white residue | 1.92 7.28 | 92% | 12% |
| Has a neutral smell | 2.48 4.16 | 84% | 52% |
| Overall satisfaction | 3.08 5.72 | 68% | 32% |
| QUESTIONNAIRE SCALE Questionnaire scale |
1 2 3 4 5 6 7 8 9
|
||
Questions rated on a scale of 1=agree completely to 9=disagree completely.
aDefined as a rating of 1, 2, or 3 on the questionnaire scale.
The patient self assessment questionnaire is not a validated tool and therefore results are exploratory and should be interpreted with caution.
Patient profiles People are different. So are our sizes.
Altreno comes in 20g and 45g sizes for different patient needs.
Patient profiles People are different. So are our sizes.
Altreno comes in 20g and 45g sizes for different patient needs.
Olivia
Aged between 18-to-25.
Curious, and looking for a low commitment option. The 20g size lasts up to 45 days, which can suit first-time users.
MSRP $60 per tube
Jennifer
25+ years old
Has tried Altreno or another tretinoin before.
The 45g size lasts about three months, which can be convenient for an experienced user.
MSRP $115 per tube
Olivia
Aged between 18-to-25.
Curious, and looking for a low commitment option. The 20g size lasts up to 45 days, which can suit first-time users.
MSRP $60 per tube
Jennifer
25+ years old
Has tried Altreno or another tretinoin before.
The 45g size lasts about three months, which can be convenient for an experienced user.
MSRP $115 per tube
Prescribing Information
Indications and Usage
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Important Safety Information
Altreno is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Skin Irritation: Patients using Altreno may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of Altreno, or discontinue use. Avoid application of Altreno to eczematous or sunburned skin.
Ultraviolet Light and Environmental Exposure: Minimize unprotected exposure to ultraviolet light, including sunlight and sunlamps. Warn patients with frequent sun exposure and those with inherent sensitivity to sunlight to exercise caution. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided.
Fish Allergies: Altreno contains soluble fish proteins. Use with caution in patients with known sensitivity or allergy to fish. Advise patients to contact their healthcare provider if they develop pruritus or urticaria.
Adverse Reactions: The most common adverse reactions in clinical trials were application site dryness (4%), pain (3%), erythema (2%), irritation (1%) and exfoliation (1%).
Nursing Women: It is not known whether topical administration of tretinoin could result in sufficient systemic absorption to produce detectable concentrations in human milk. The developmental health benefits of breastfeeding should be considered along with the mother’s clinical need for Altreno and any potential adverse effects on the breastfed child from Altreno.
To report suspected adverse reactions, contact Customer Service at 1-800-321-4576 or the FDA at 1-800-FDA-1088 or fda.gov/medwatch.
How Do I Get Altreno?
Altreno is a powerful Rx product, which means it requires a prescription. We can help you get in touch with a healthcare provider in two easy ways:
1. Online consultation
2. In-person visit