Indications and Usage
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Important Safety Information
Altreno is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Skin Irritation: Patients using Altreno may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of Altreno, or discontinue use. Avoid application of Altreno to eczematous or sunburned skin.
Ultraviolet Light and Environmental Exposure: Minimize unprotected exposure to ultraviolet light, including sunlight and sunlamps. Warn patients with frequent sun exposure and those with inherent sensitivity to sunlight to exercise caution. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided.
Fish Allergies: Altreno contains soluble fish proteins. Use with caution in patients with known sensitivity or allergy to fish. Advise patients to contact their healthcare provider if they develop pruritus or urticaria.
Adverse Reactions: The most common adverse reactions in clinical trials were application site dryness (4%), pain (3%), erythema (2%), irritation (1%) and exfoliation (1%).
Nursing Women: It is not known whether topical administration of tretinoin could result in sufficient systemic absorption to produce detectable concentrations in human milk. The developmental health benefits of breastfeeding should be considered along with the mother’s clinical need for Altreno and any potential adverse effects on the breastfed child from Altreno.
To report suspected adverse reactions, contact Customer Service at 1-800-321-4576 or the FDA at 1-800-FDA-1088 or fda.gov/medwatch.
Indications and Usage
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Indications and Usage
Important Safety Information
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Important Safety Information
Altreno is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Skin Irritation: Patients using Altreno may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of Altreno, or discontinue use. Avoid application of Altreno to eczematous or sunburned skin.
Ultraviolet Light and Environmental Exposure: Minimize unprotected exposure to ultraviolet light, including sunlight and sunlamps. Warn patients with frequent sun exposure and those with inherent sensitivity to sunlight to exercise caution. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided.
Adverse reactions reported by ≥1% of subjects treated with Altreno
In 2 randomized, double-blind, vehicle-controlled trials, subjects age 9 years and older applied ALTRENO or vehicle once daily for 12 weeks. The majority of subjects were White (74%) and female (55%). Approximately 47% were Hispanic/Latino and 45% were younger than 18 years of age. Adverse reactions reported by ≥1% of subjects treated with ALTRENO and more frequently than vehicle are summarized below.
Altreno (n=767)
Vehicle (n=783)
Application-site dryness
4%
< 1%
Application-site pain*
3%
< 1%
Application-site erythema
2%
< 1%
Application-site irritation
1%
< 1%
Application-site exfoliation
1%
< 1%
*Application-site pain was defined as application-site stinging, burning, or pain.
Application site tolerability reactions at any post baseline visit
Skin irritation was evaluated by active assessment of erythema, scaling, hypopigmentation, hyperpigmentation, itching, burning and stinging. The percentage of subjects who were assessed to have these signs and symptoms at any post-baseline visit are summarized below.
Mild / Moderate / Severe
Altreno (n=760)
Vehicle (n=782)
Erythema
51%
44%
Scaling
49%
30%
Hypopigmentation
12%
10%
Hyperpigmentation
35%
35%
Itching
35%
28%
Burning
30%
14%
Stinging
21%
8%
Treatment-emergent AE's (TAE) in moderate/severe treatment groups
Treatment related AEs reported by ≥1% patients
Altreno 0.05% lotion
Moderate adult female patients (n=240)
Altreno 0.05% lotion
Severe adult female patients (n=22)
Application site pain
7 (2.9%)
1 (4.5%)
Application site
dryness
12 (5.0%)
1 (4.5%)
Application site
erythema
4 (1.7%)
0 (0.0%)
Application site
exfoliation
3 (1.3%)
0 (0.0%)
Application site
pruritus
4 (1.7%)
0 (0.0%)
to treat your patients' acne.*
Prescribing Information
Indications and Usage
Altreno® (tretinoin) Lotion, 0.05% is indicated for the topical treatment of acne vulgaris in patients 9 years of age and older.
Important Safety Information
Altreno is for topical use only. Not for ophthalmic, oral, or intravaginal use.
Skin Irritation: Patients using Altreno may experience application site dryness, pain, erythema, irritation, and exfoliation. Depending upon the severity of these adverse reactions, instruct patients to use a moisturizer, reduce the frequency of the application of Altreno, or discontinue use. Avoid application of Altreno to eczematous or sunburned skin.
Ultraviolet Light and Environmental Exposure: Minimize unprotected exposure to ultraviolet light, including sunlight and sunlamps. Warn patients with frequent sun exposure and those with inherent sensitivity to sunlight to exercise caution. Use sunscreen and protective clothing over treated areas when exposure cannot be avoided.
Fish Allergies: Altreno contains soluble fish proteins. Use with caution in patients with known sensitivity or allergy to fish. Advise patients to contact their healthcare provider if they develop pruritus or urticaria.
Adverse Reactions: The most common adverse reactions in clinical trials were application site dryness (4%), pain (3%), erythema (2%), irritation (1%) and exfoliation (1%).
Nursing Women: It is not known whether topical administration of tretinoin could result in sufficient systemic absorption to produce detectable concentrations in human milk. The developmental health benefits of breastfeeding should be considered along with the mother’s clinical need for Altreno and any potential adverse effects on the breastfed child from Altreno.
To report suspected adverse reactions, contact Customer Service at 1-800-321-4576 or the FDA at 1-800-FDA-1088 or fda.gov/medwatch.
How Do I Get Altreno?
Altreno is a powerful Rx product, which means it requires a prescription. We can help you get in touch with a healthcare provider in two easy ways:
1. Online consultation
2. In-person visit
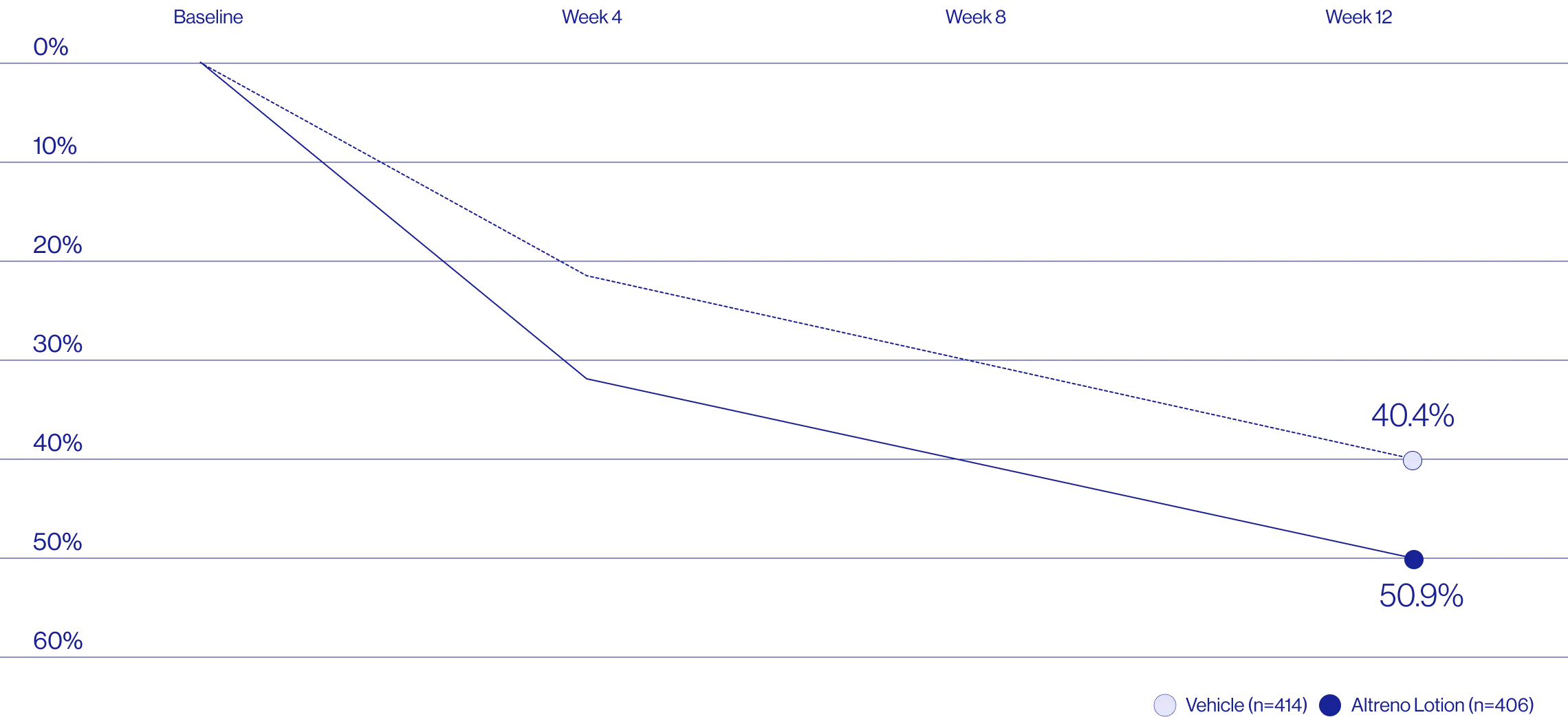
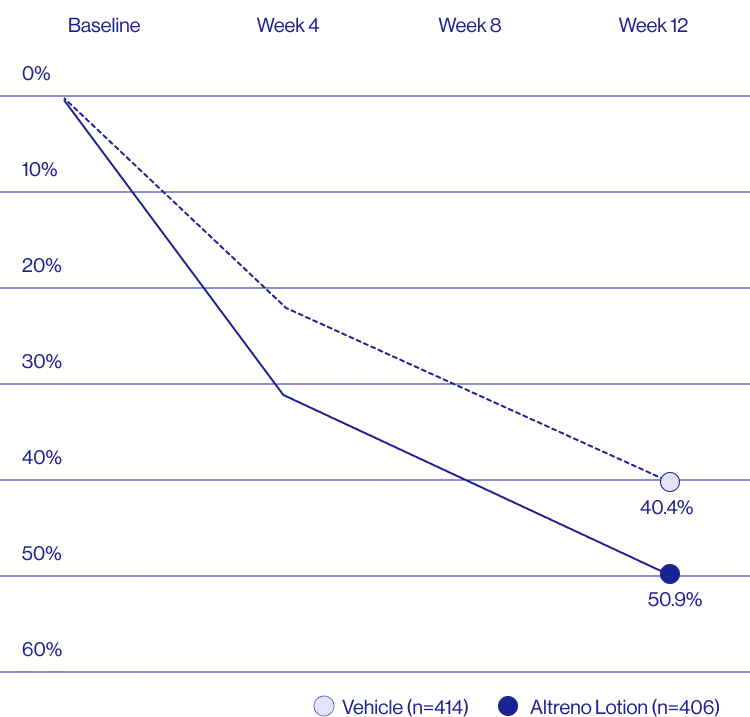
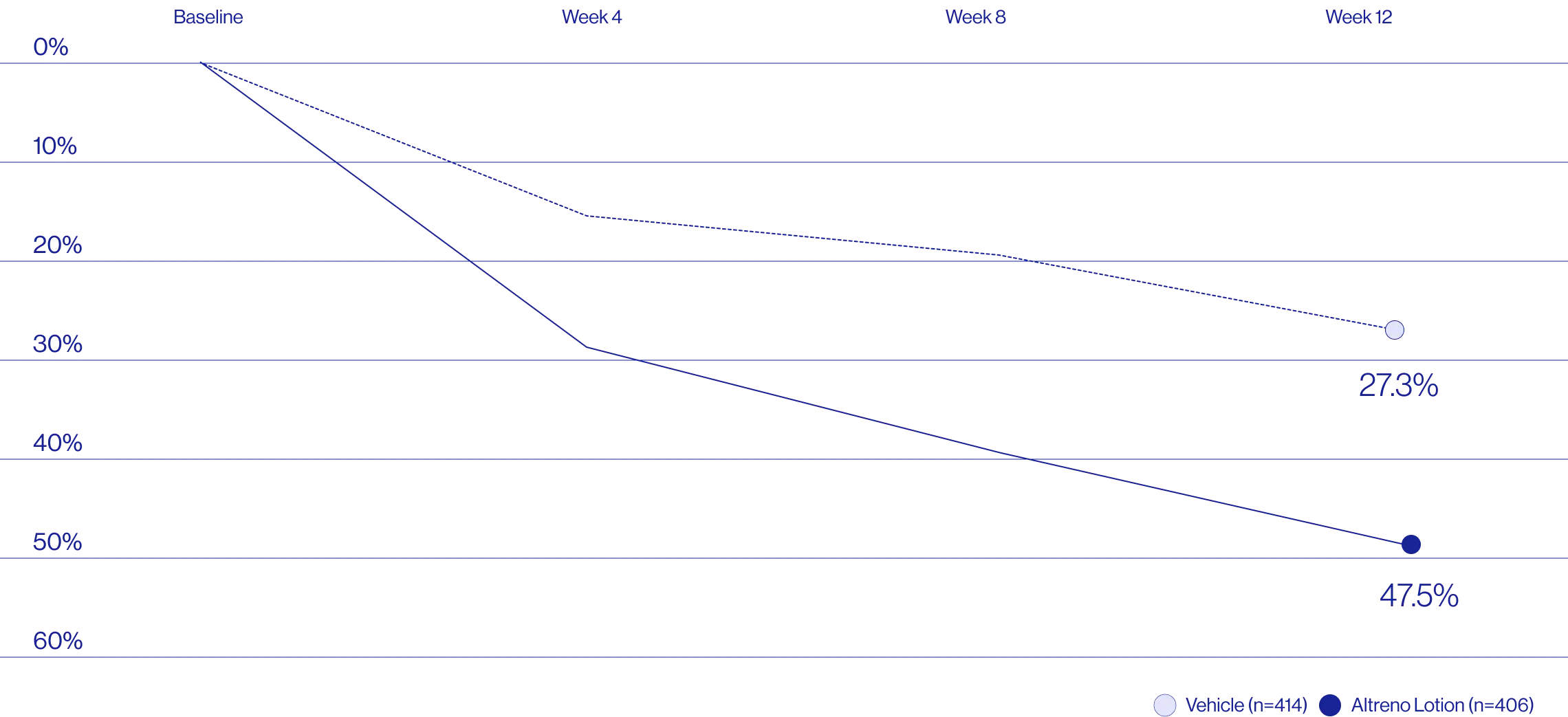
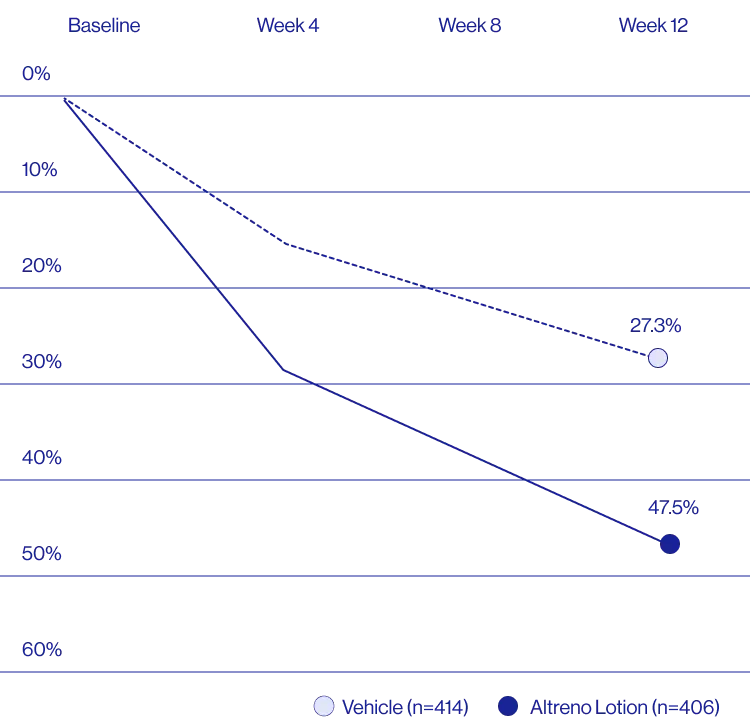
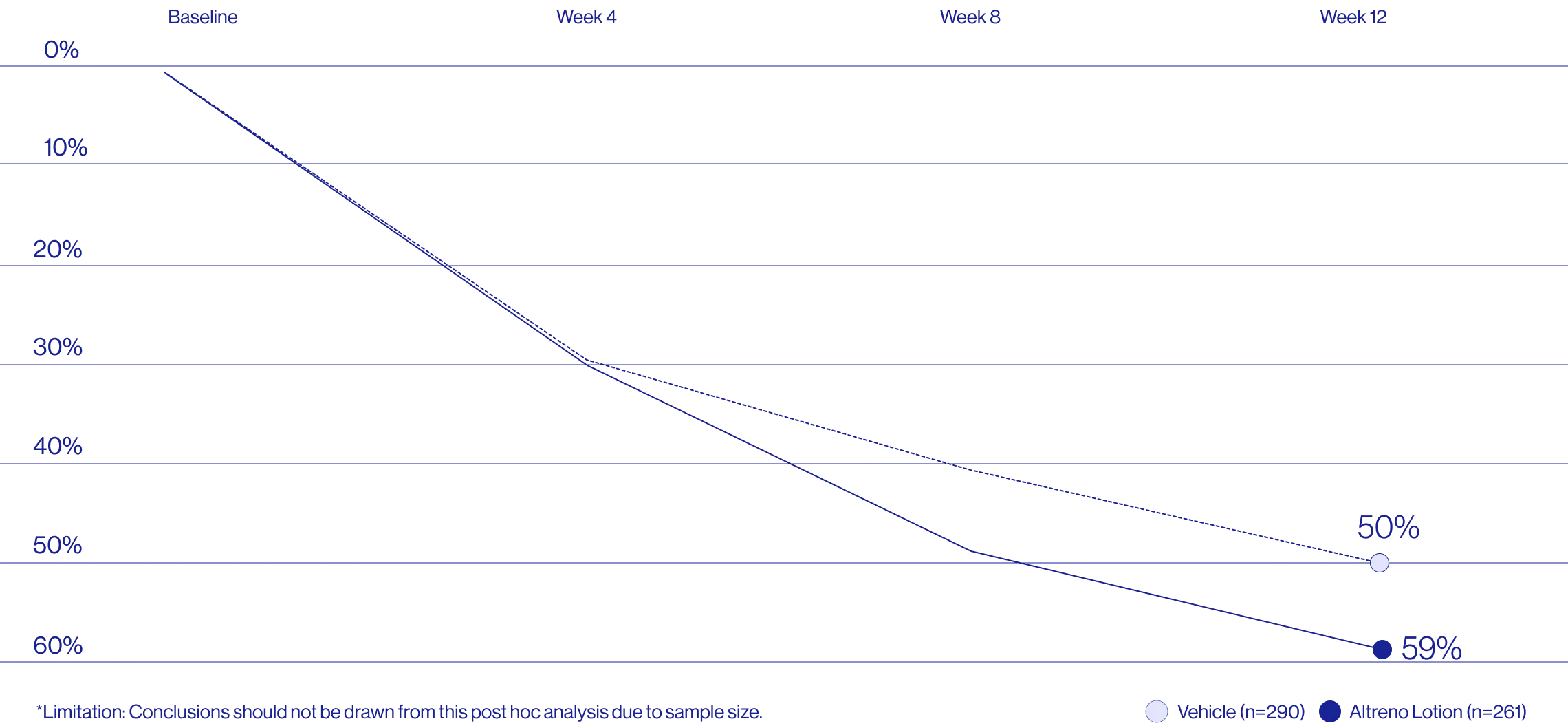
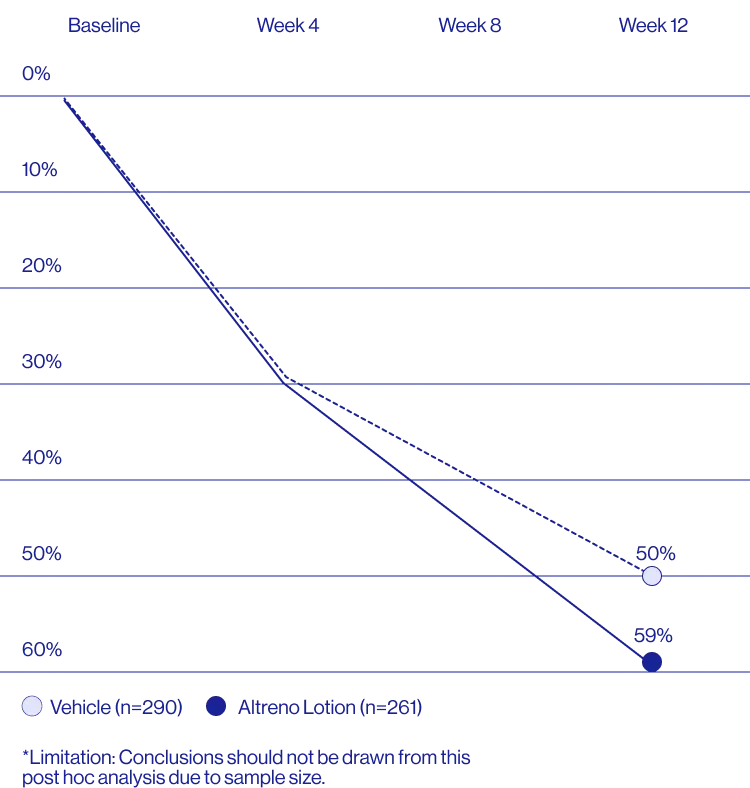
Study Design
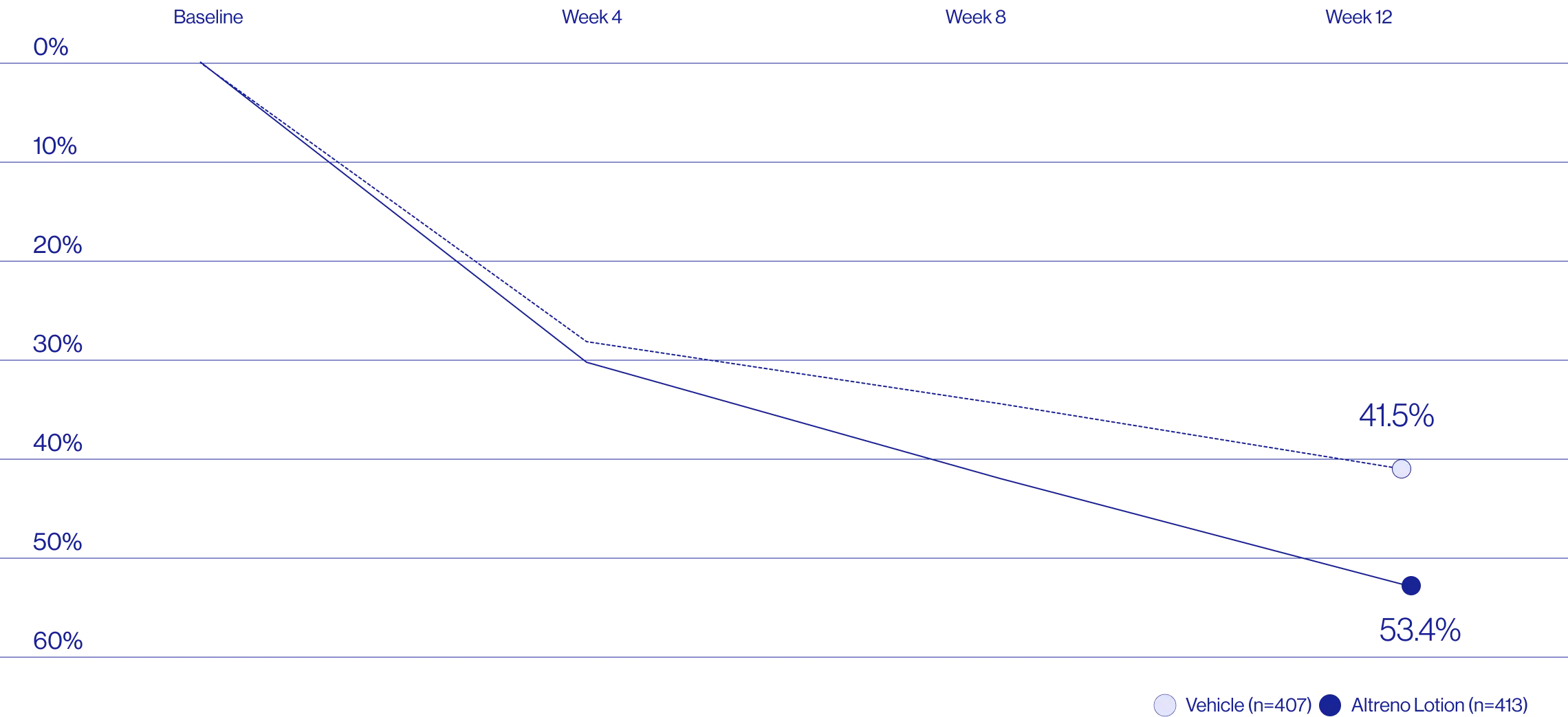
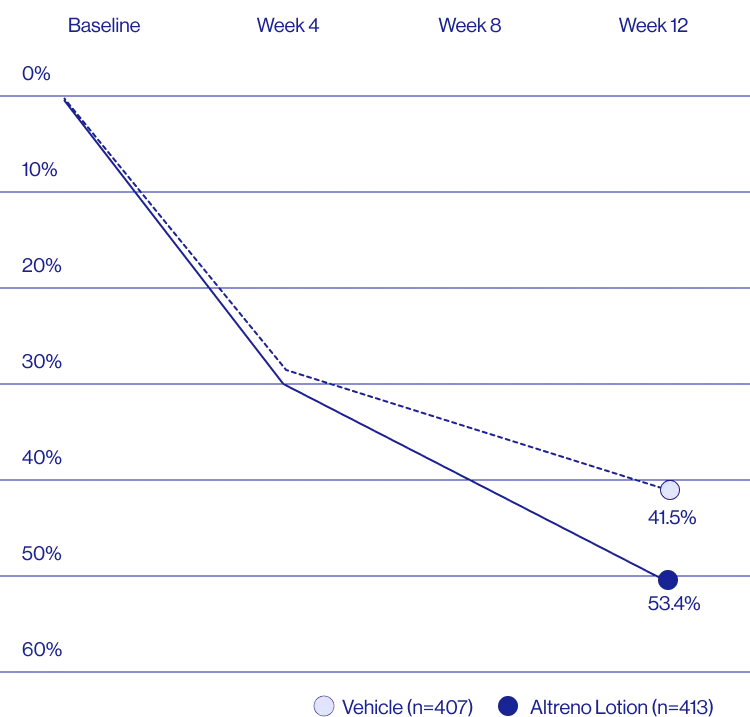
Treatment success was defined as at least a 2-grade improvement from baseline and an Evaluator's Global Severity Score of clear (0) or almost clear (1). Results of 2 phase 3, multicenter, randomized, double-blind, vehicle-controlled, parallel-group studies comparing the efficacy and safety of Altreno lotion once daily for 12 weeks with vehicle in patients 9 years and older with moderate-to-severe acne vulgaris (baseline 20 to 40 inflammatory lesions). The efficacy of once daily use of ALTRENO for the treatment of acne vulgaris were assessed in two multicenter, randomized, double-blind clinical trials enrolling 1640 subjects age 9 years and older with acne vulgaris. Enrolled subjects had a score of moderate (3) or severe (4) on the Evaluator’s Global Severity Score (EGSS), 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 non-inflammatory lesions (open and closed comedones) and two or fewer facial nodules. The coprimary efficacy endpoints of success on the EGSS, absolute change in non-inflammatory lesion count, and absolute change in inflammatory lesion count were assessed at Week 12. Success on the EGSS was defined as at least a 2-grade improvement from Baseline and an EGSS score of clear (0) or almost clear.
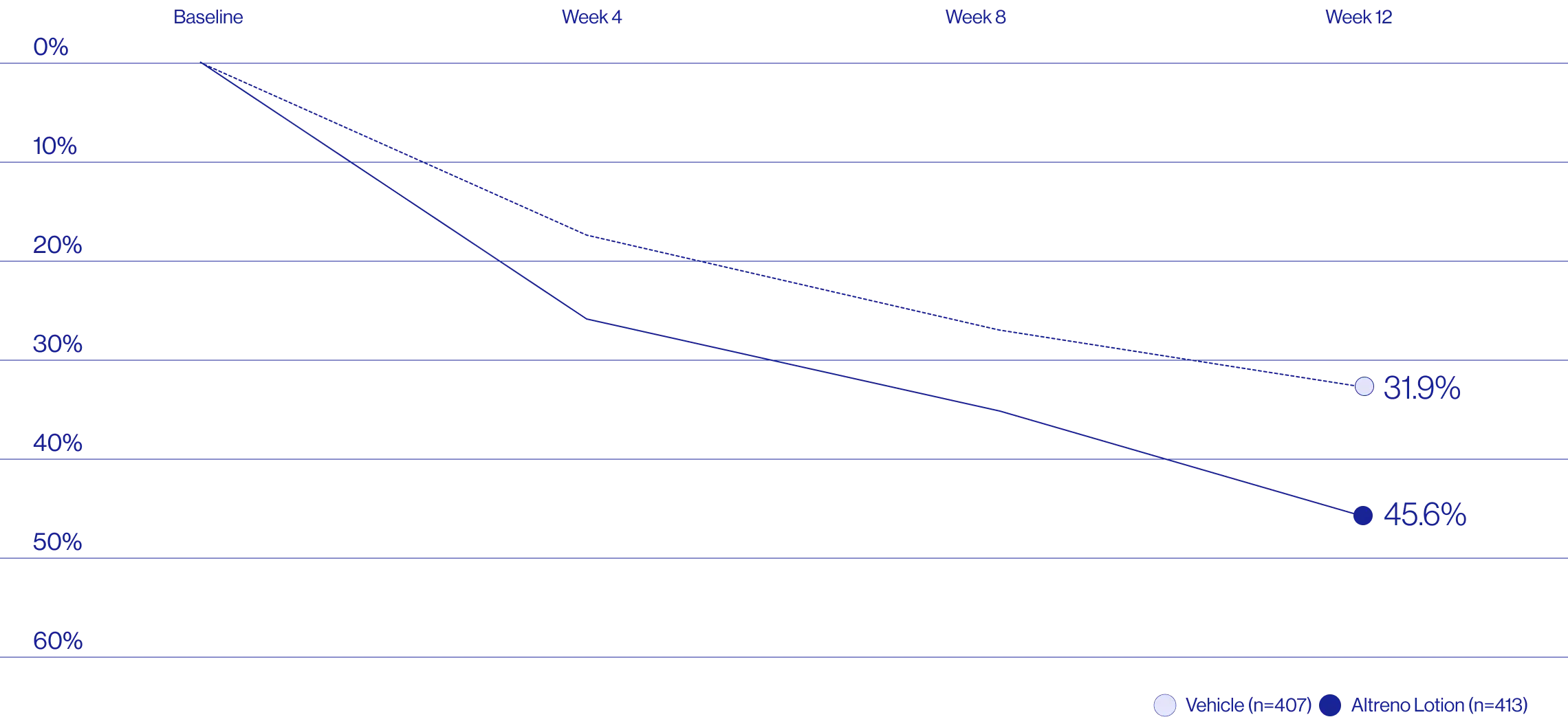
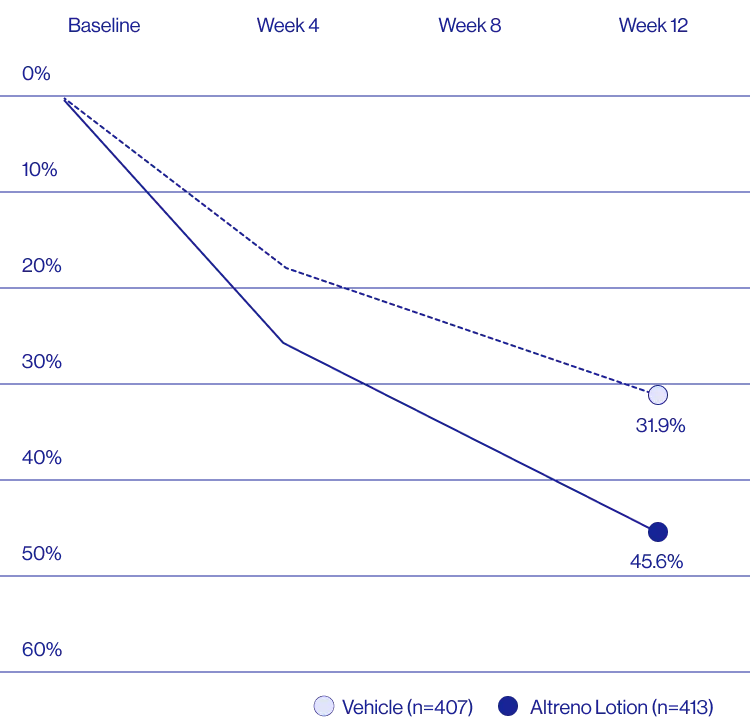
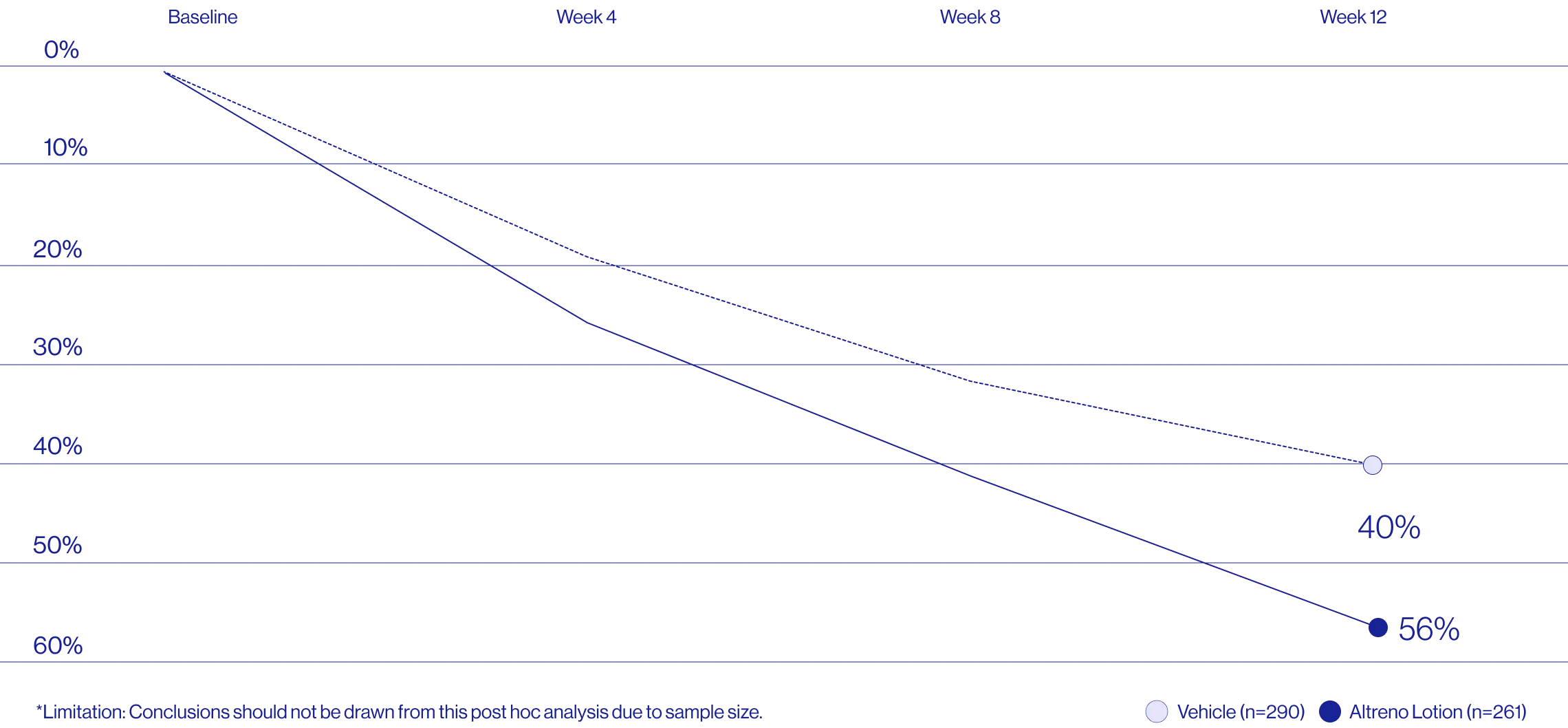
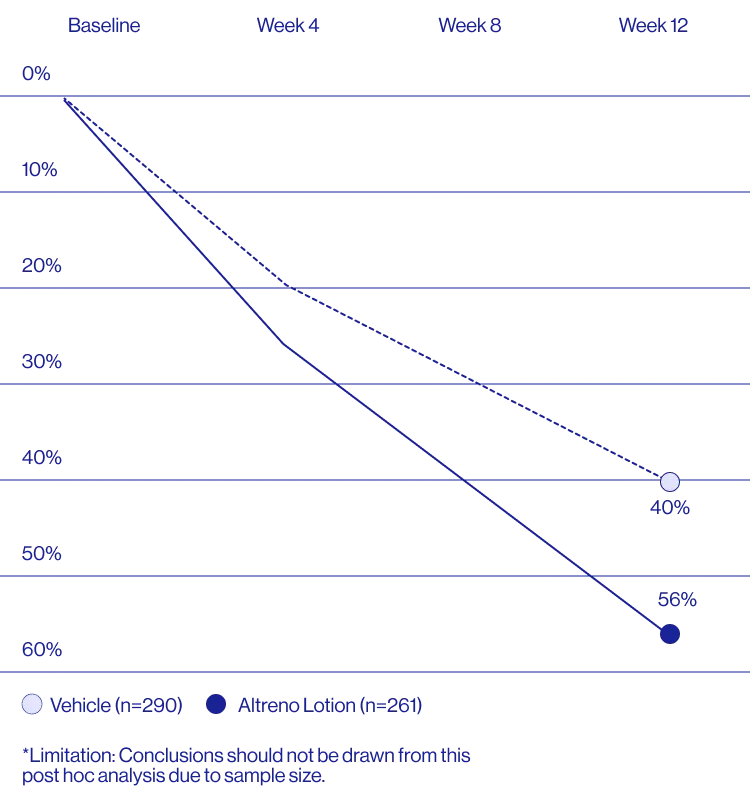
Study Design
A post hoc analysis of 606 adult female patients from 2 multicenter, randomized, double-blind, vehicle-controlled, parallel group clinical studies (Tyring et al. 2018) in patients with moderate or severe acne was conducted. Patients eligible for the post hoc analysis were adult female patients aged 18 to 58 years who presented with 20 to 40 inflammatory lesions (papules, pustules, and nodules), 20 to 100 non-inflammatory lesions (open and closed comedones), and two nodules or less; and an Evaluator Global Severity Score [EGSS] score of 3 (moderate) or 4 (severe). Patients were treated with either Altreno (n=236) or vehicle (n=258).