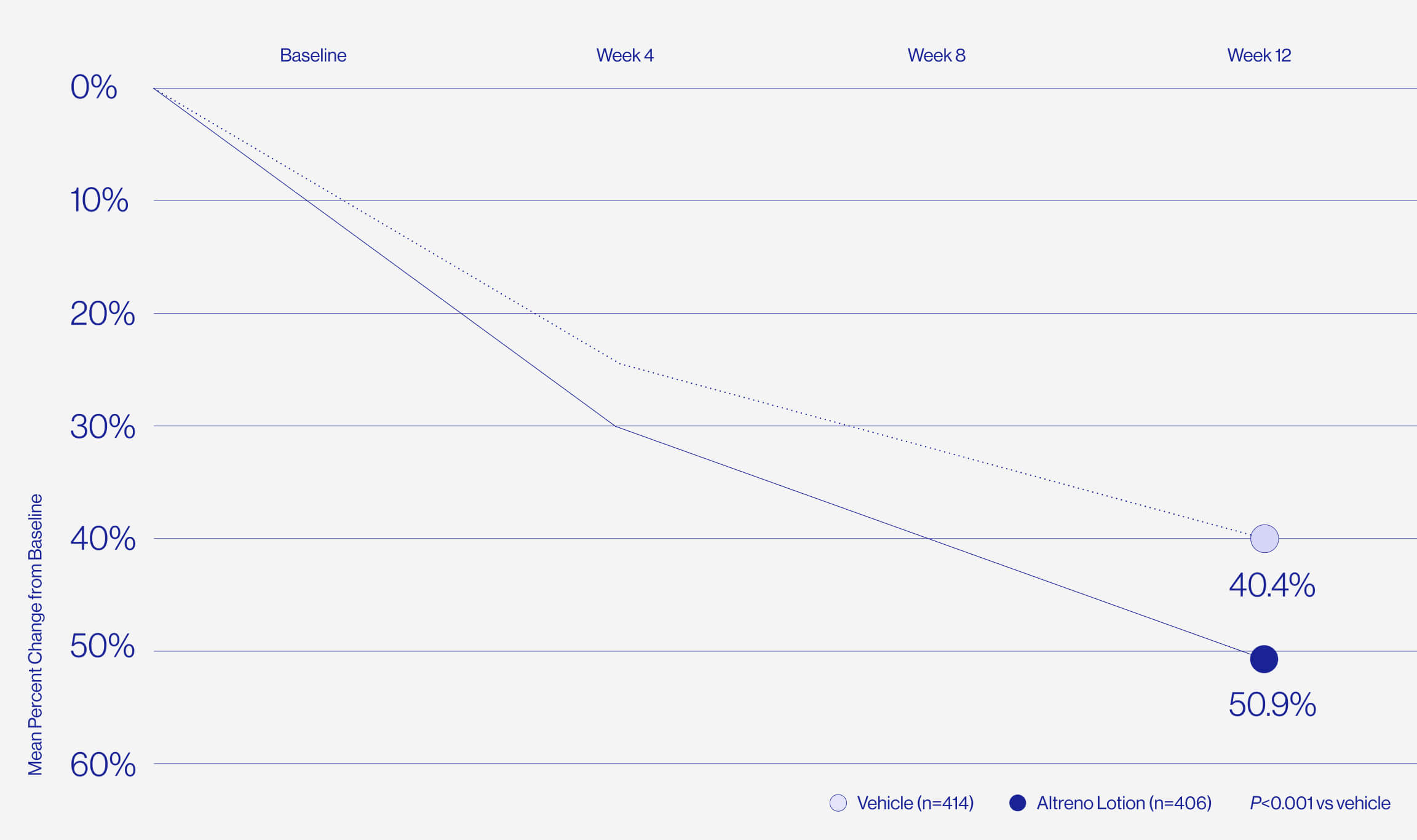
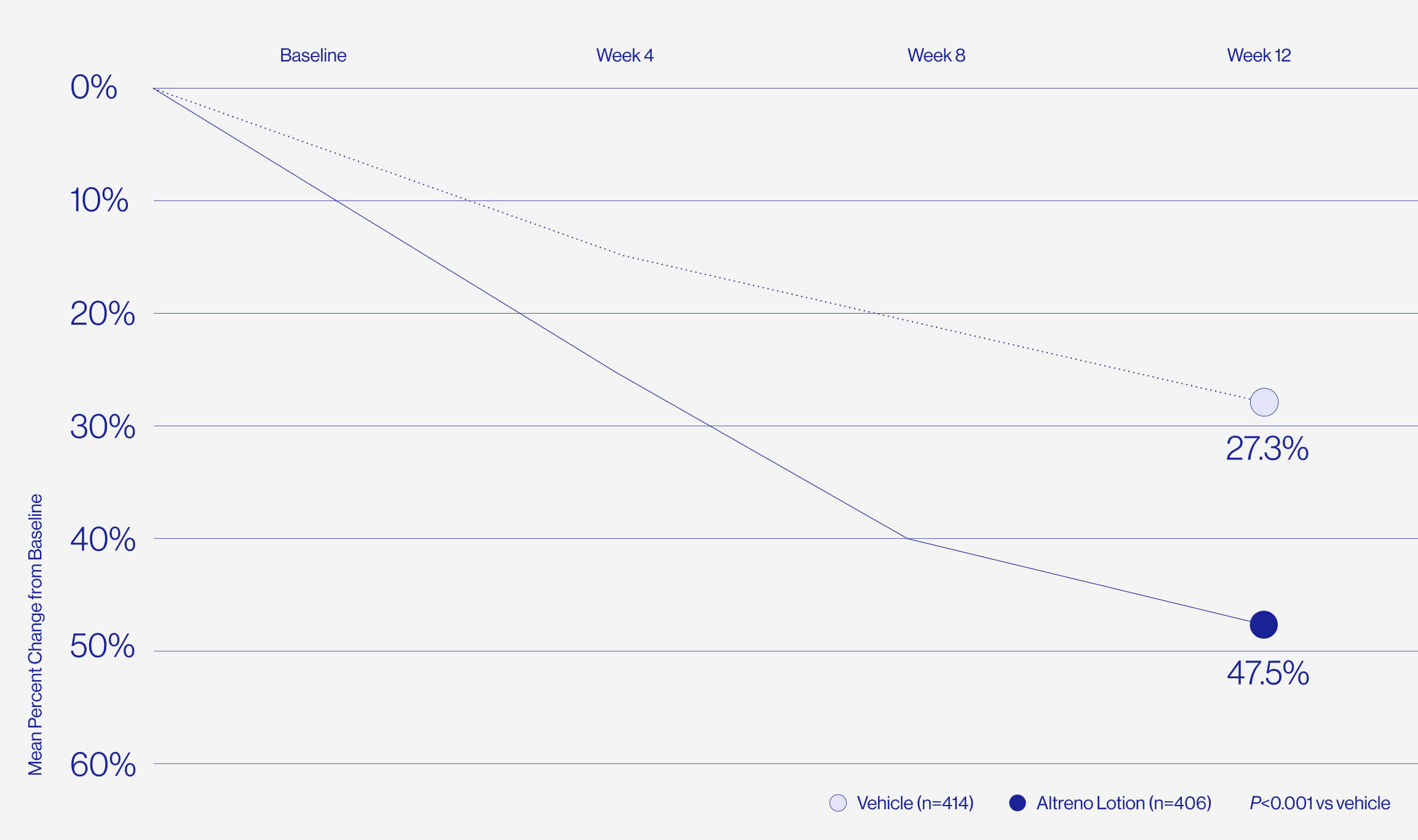
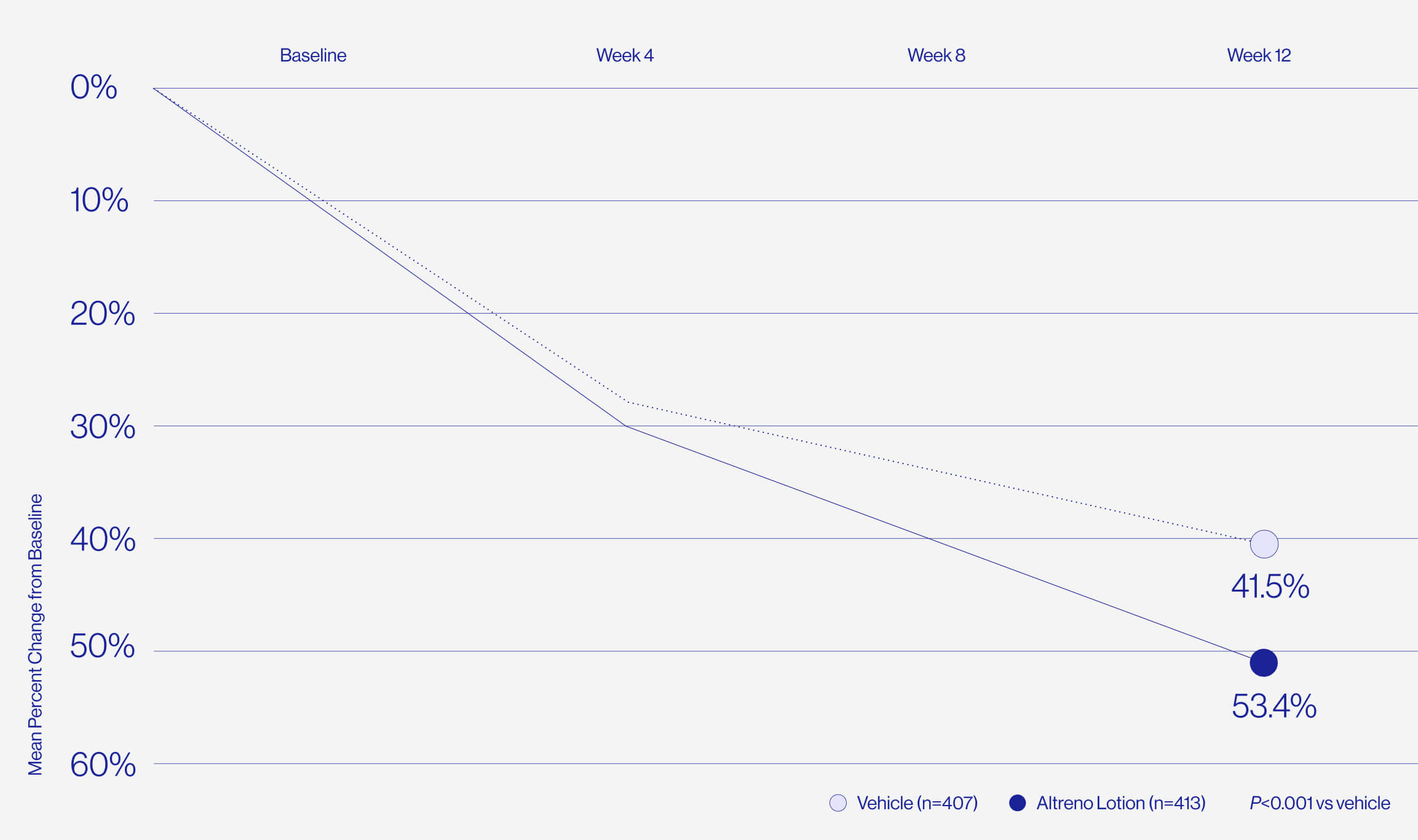
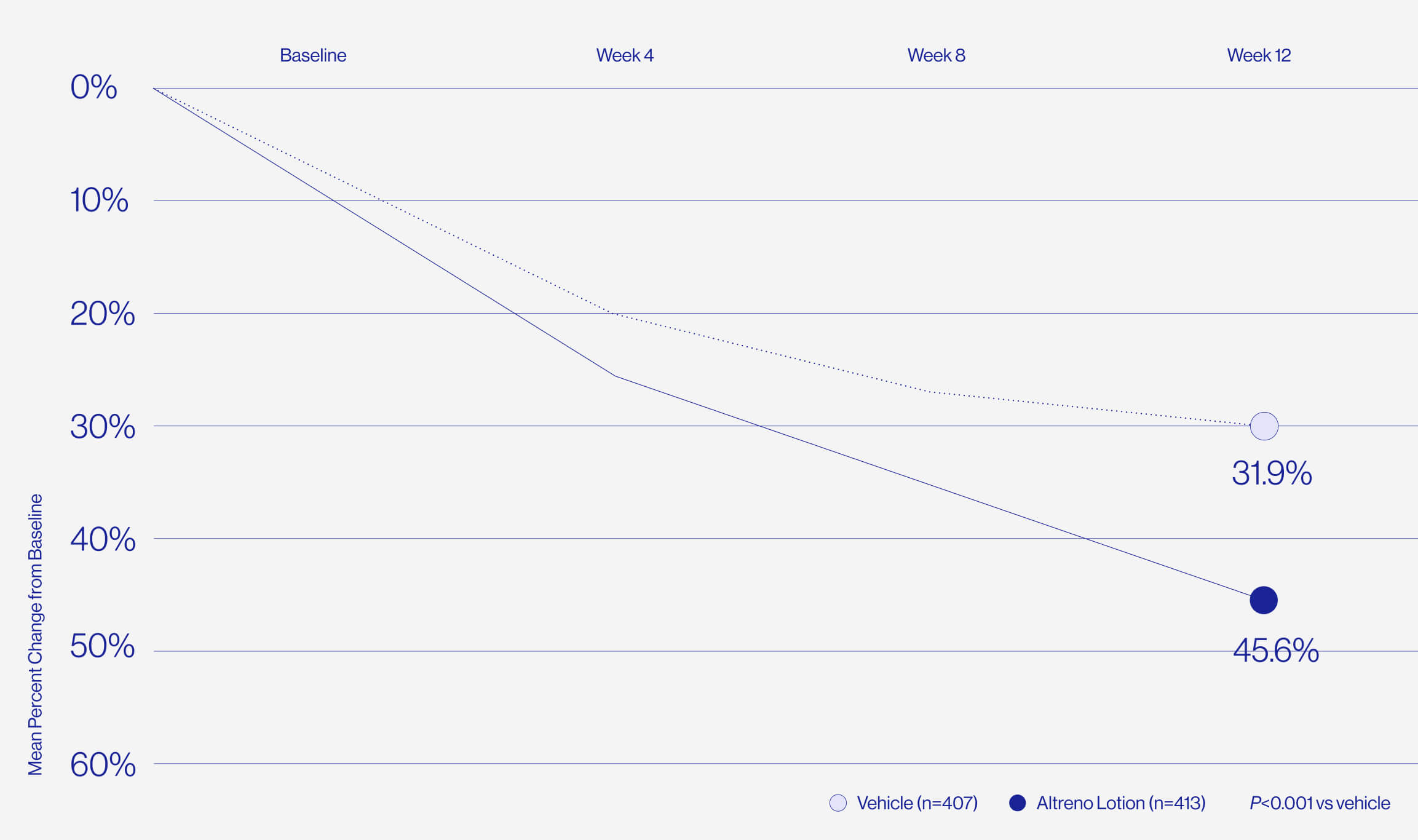
Only 1% of patients reported
skin irritation in clinical trials.
For patients who are worried about dryness and irritation comes the only tretinoin in a hydrating lotion formulation, delivered by a unique polymeric honeycomb mesh delivery system.